Scantox Group
Scantox, a contract research organization (CRO), has developed a significant footprint in toxicology and preclinical lead optimization over the past few decades. By leveraging scientific expertise and a flexible service model, Scantox has remained at the forefront of innovation, providing tailored solutions to meet the evolving needs of a diverse client base.
Our services support a broad spectrum of preclinical drug development elements. From lead optimization within in vitro and in vivo efficacy models, formulation development, regulatory toxicology studies incl. supportive functions as clinical pathology, bioanalysis, histology all the way to manufacturing and distribution of products for clinical trial phase 1 and 2. This enables clients to progress their development projects under one roof in a trustworthy collaborative matter based on the highest technical and scientific standards.
Our client base ranges from Start-ups, to pharmaceutical companies of all sizes, biotechs and CDMOs.
Core Competencies
Scantox is specialized in preclinical contract research services. Our work enables pharmaceutical and biotechnology companies to progress drug development projects, based on solid high-quality data. Core competencies within the Scantox Group include:
- Explorative and efficacy studies
- PK-studies
- General toxicology including Juvenile & EFD
- Local tolerance
- Wound healing
- Vaccines
- GLP and GMP compliant
Key Specialities
In addition, each of our locations offer a wide range of unique key specialties:
- Infectious disease models (BSL 1-3)
- Stereotactic surgery
- Advanced imaging PET-CT; PET-NMR, MRI
- Oncology, xenograft models
- Metabolic disorders
- Customized animal models incl. orphan diseases
- Juvenile minipig studies incl. feeding studies
- DART in minipigs
- Colony animals
- Safety pharmacology studies
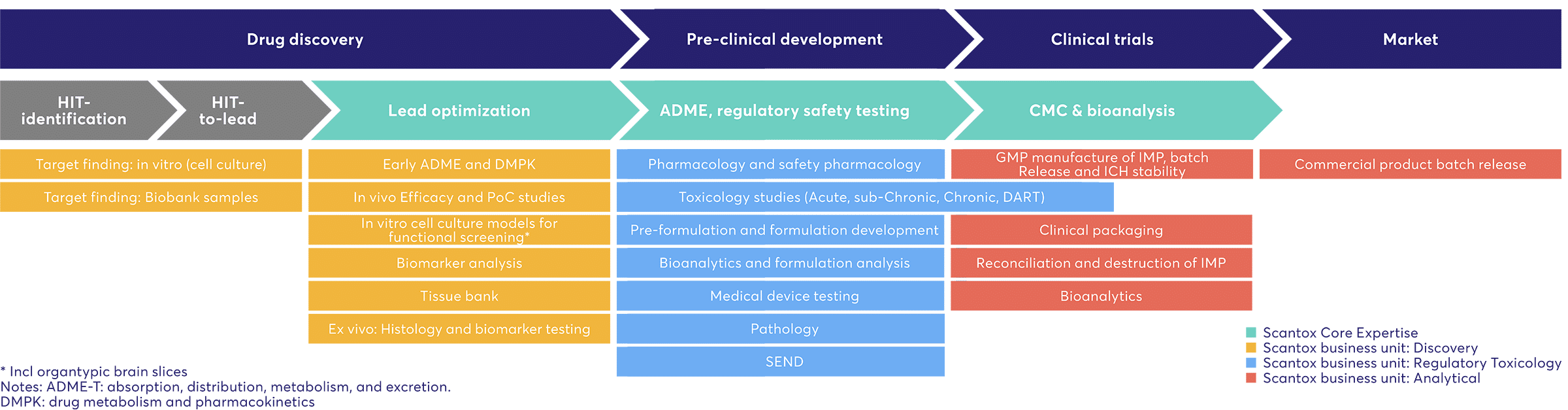
Scantox Services within the Drug Development Process
The drug and device development process timeline can be divided into different lifecycle phases, and it is often a 10+ years process for a new drug to reach the intended market. Once a lead compound is identified within the drug discovery phase, the preclinical phase of drug development begins with in vivo research to determine the efficacy and safety of the drug. Preclinical trials test the new drug on non-human subjects for efficacy, toxicity, and pharmacokinetic (PK) information. Scantox’ core business is studies within the Preclinical phase, but we also extend our expertise into in vitro and in vivo studies in early discovery. Scantox is catered towards providing services for both the biopharmaceutical innovative SMEs, as well as the bigger pharma companies on the global arena.Scantox is your guarantee for solid timelines, on-time and on-budget deliveries, the highest quality scientific data, and a close partnership where you are in contact with a dedicated scientist from start to finish of your study.
GLP Accredited Company
Scantox is your GLP-accredited CRO partner.
The fundamental purpose of the Principles of Good Laboratory Practice (GLP) is to ensure the quality and integrity of test data related to non-clinical studies. The way in which study data is generated, handled, reported, retained and archived has continued to evolve in line with the introduction and ongoing development of supporting technologies. However, the main purpose of the requirements of the Principles of GLP remains the same in having confidence in the quality, the integrity of the data and being able to reconstruct activities performed during the conduct of non-clinical studies.
International Service
Scientific data from the Scantox Group is From the corporate headquarters in Denmark, Scantox is today serving customers all over the world. And the company is world known for its high quality data, on time deliveries, and high ethical standards.
In 2022 Scantox’ international journey began, adding not only new geographical locations, but also an array of complimentary services to the company’s study portfolio.
Locations
Headquarters of the Scantox Group is located in Denmark, in Ejby near Køge. Subsidiaries include:
Denmark: Ballerup
Sweden: Lund, Solna and Gothenburg
Austria: Grambach