Leading Expert in Toxicology Studies with Göttingen Minipigs
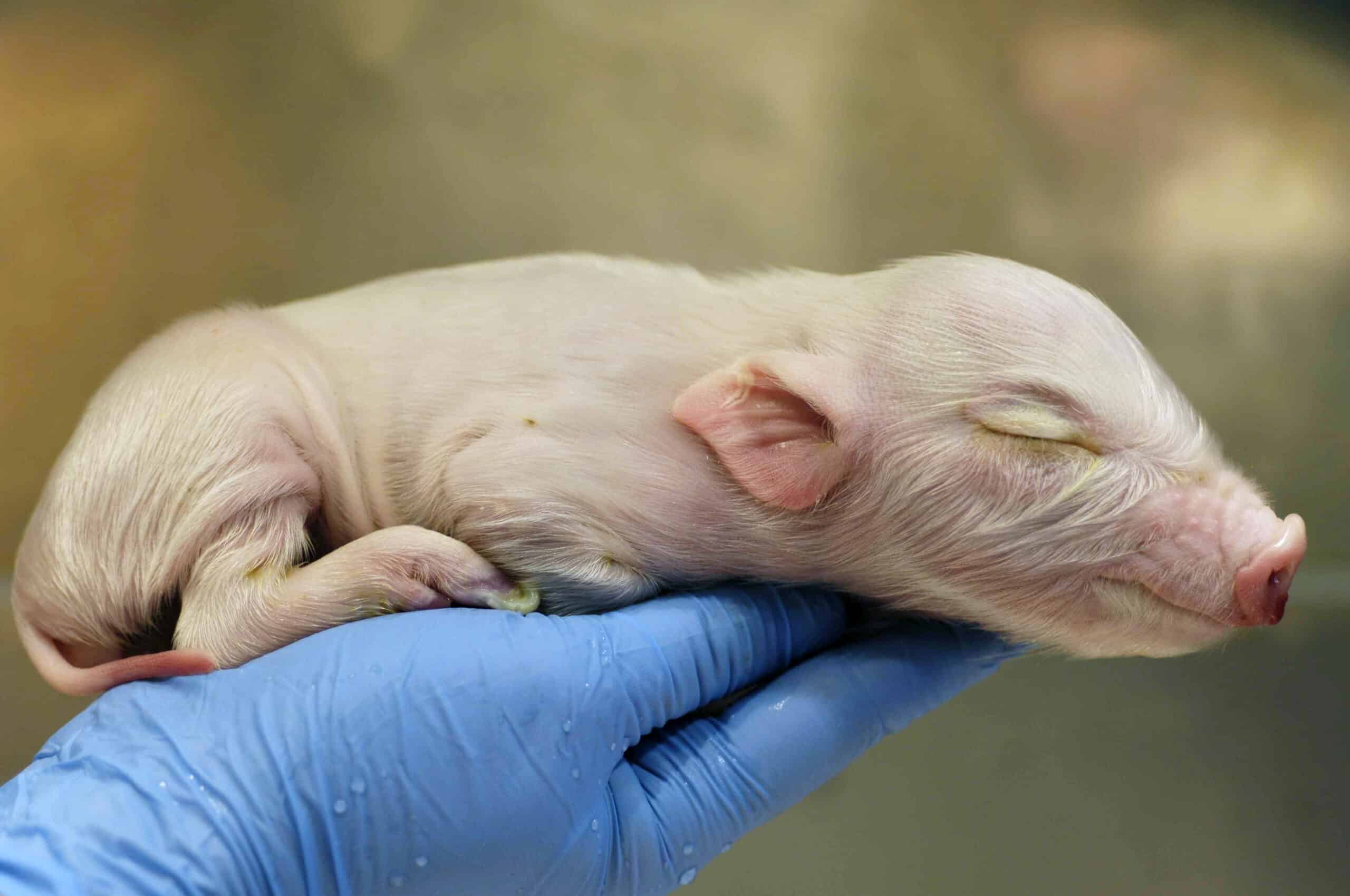
Scantox is considered world leading within the field of toxicology studies with Göttingen Minipigs. Testing on juvenile animals is required to support the use of formulations within the pediatric field.
Multiple parameters can be investigated in a juvenile minipig toxicology study, including bioavailability of the test item, clinical observation, food consumption, crown-rump evaluation and neurological examination.
Since 2007
Scantox has performed minipig toxicology studies since 2007.
As the leading CRO within this field we have a uniquely broad experience in various types of juvenile toxicology studies including oral bioavailability studies in juvenile minipigs, investigation of pharmacokinetic profile in lactating sows and juvenile minipigs after dosing of sows as well as dermal application of test item on juvenile minipigs.
Additionally, we have a setup for juvenile minipig feeding studies, where a test item is added in milk replacement to allow feeding of the juvenile animals from post-natal day 2.
